The Periodic Table of Elements
We have learned how to draw pictures of atoms by placing electrons in shells starting next to the nucleus and moving outwards. Why have we done this? When we do this we discover how many electrons there are in the outer shell. This is important because:
- When atoms of different elements react they have to collide. They need to get get up close and personal.
- When they collide it is the outer shells of electrons that come together.
Drawing atoms is time consuming but because we are only interested in the outer electrons we can simplify the process.
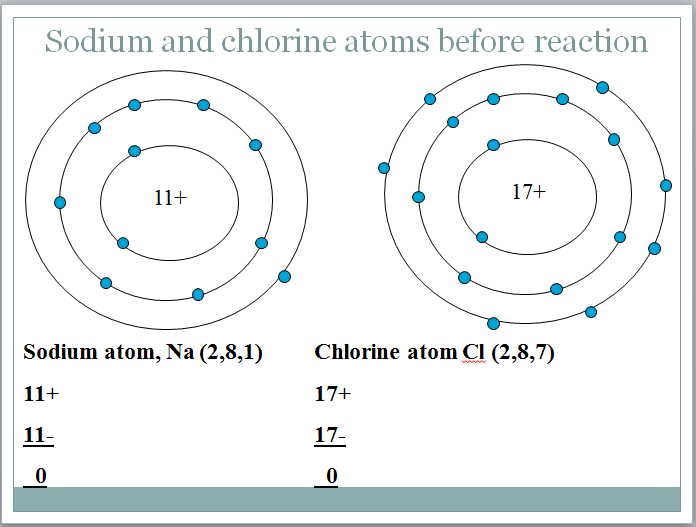
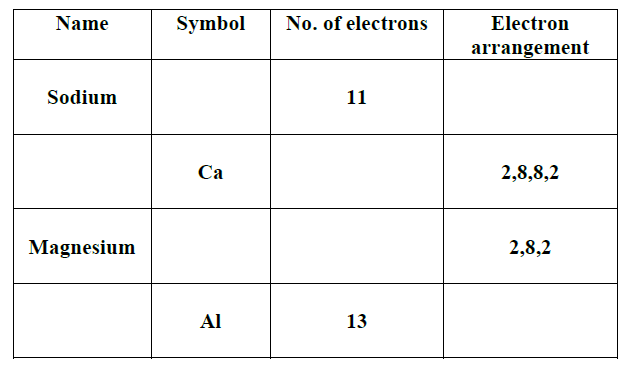
Sodium has an atomic number of 11. The sodium atom has has 11 protons in its nucleus and eleven electrons in shells around it.
Two electrons fill the first shell. The next shell is also full with 8 electrons and the third shell has the last remaining electron. Instead of drawing a picture of the atom it’s simpler to write down how many electrons there are in each shell. This is known as the electron configuration of an atom.
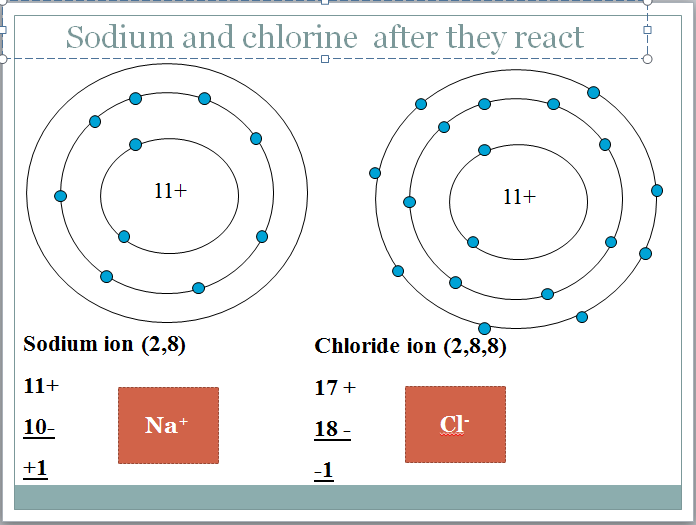
The electron configuration of the sodium atom is: Na (2.8,1)
The electron configuration of the potassium atom is: K (2,8,8,1)
Vertical columns in the periodic table are known as groups. Lets use our knowledge of atoms and their electrons
Group 1 The alkali metals
Lithium, Sodium, Potassium, Rubidium, Caesium and Frankium are highly reactive metals that have to be stored free of air or moisture. (check out the Brainiac video in the the prezi a few posts back). They are silver coloured when freshly cut. The electron configurations:
Lithium atomic number 3, Li (2.1)
Sodium atomic number 11, Na (2,8,1)
Potassium atomic number 19 K (2.8,8,1)
All the group 1 alkali metals have 1 electron in the outer shell
Group 17 The Halogens
Fluorine atomic number 9, pale yellow gas with electronic configuration F (2,7) Highly reactive.
Chlorine atomic number 17, Pale green gas with electronic configuration Cl (2,8,7) Highly reactive
Bromine, Brown volatile liquid that gives of a red, brown gas with electronic configuration Br (complicated but 7 electrons in its outer shell). Very reactive.
The elements in group 17 have similar properties and they all have 7 electrons in their outer shell.
Group 18 the inert Gases
Helium atomic number 2, electronic configuration He (2) very unreactive
Neon atomic number 10, electronic configuration Ne (2,8) very unreactive
Argon atomic number18 electronic configuration Ar (2,8,8) very unreactive.
The inert gases glow when they are placed in a tube and a large voltage is applied. They all have a full outer shell of electrons
Homework.
Test your understanding of electron configuration. Use the periodic table at the top of the post to find atomic numbers and symbols
Question 1. Find these elements on the periodic table, carbon, aluminium, Calcium, Phosphorus, Magnesium, oxygen. Write down their chemical symbol followed by the electron configuration. The first one is done for you.
Carbon C (2,4)
Question 2 Here are the electron configurations of a number of elements. Work out the atomic number and identify the element using the periodic table.
electronic configurations:
(2,5)
(2,8,5)
(2,8,4)
(2,8,3)
(2,2)
The first one is done for you
(2,5), the atomic number is 7 and the chemical symbol is N