So much for atoms what about ions?
When we started this topic the big question was " what happens when atoms react"?When we started this topic the big question was ” what happens when atoms react”? We know enough now to answer this. Here is a review of the main points so far.
- Elements with same number of outer electrons are arranged in columns on the Periodic Table called groups. Elements with the same number of electrons in their outer shell tend to have similar physical and chemical properties.
- Finally atoms gain lose or share electrons to achieve a full outer shell like the inert gases.Now lets have a look at what happens when sodium and chlorine atoms react.
Sodium burns fiercely in an atmosphere of chlorine.
The product is a white solid, sodium chloride or ordinary table salt.
This compound is harmless.What has happened to the sodium and chlorine atoms?
Sodium burns fiercely in an atmosphere of chlorine.
The product is a white solid, sodium chloride or ordinary table salt.
This compound is harmless.
What has happened to the sodium and chlorine atoms?
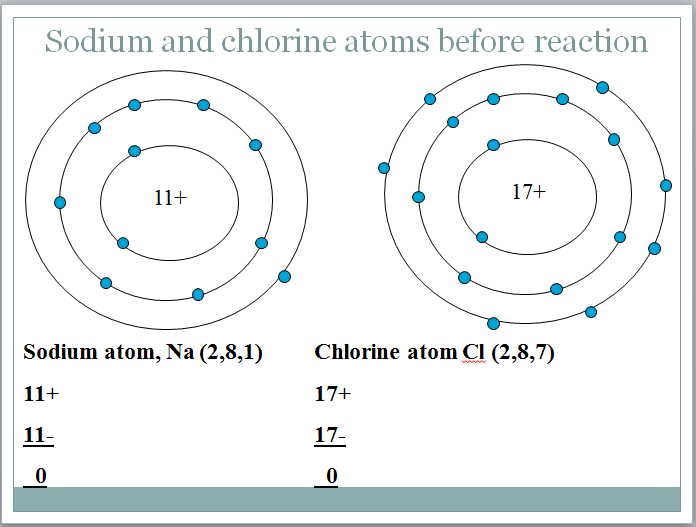
Can you spot the deliberate mistake in the diagram?
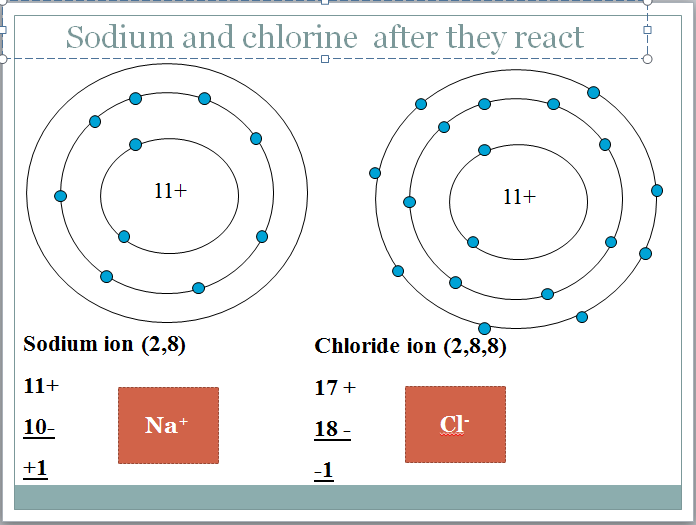
The sodium atom has lost an electron to become a positively charged ion.
The chlorine atom has gained an electron to become a negatively charged chloride ion.
Watch sodium burning violently in chlorine here.

China professional silicone kitchen utensils supplier
Wholesale Best Semi-automatic Cartridge Filling Machie