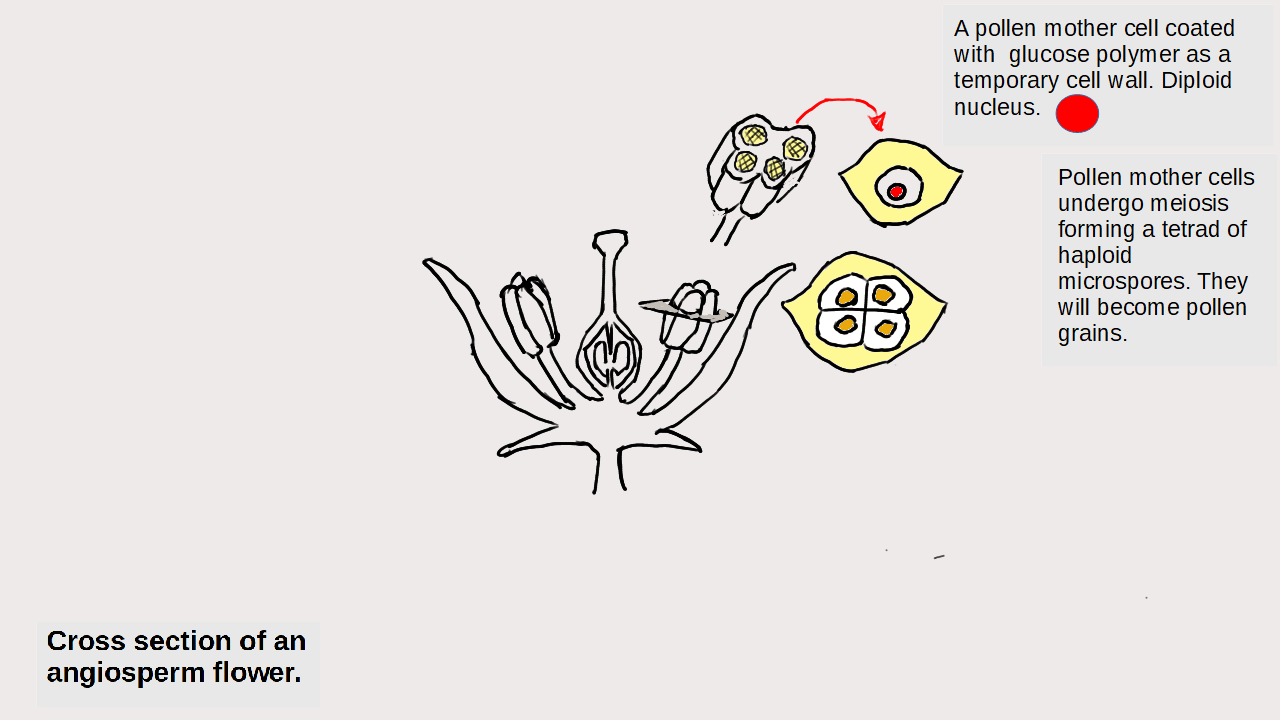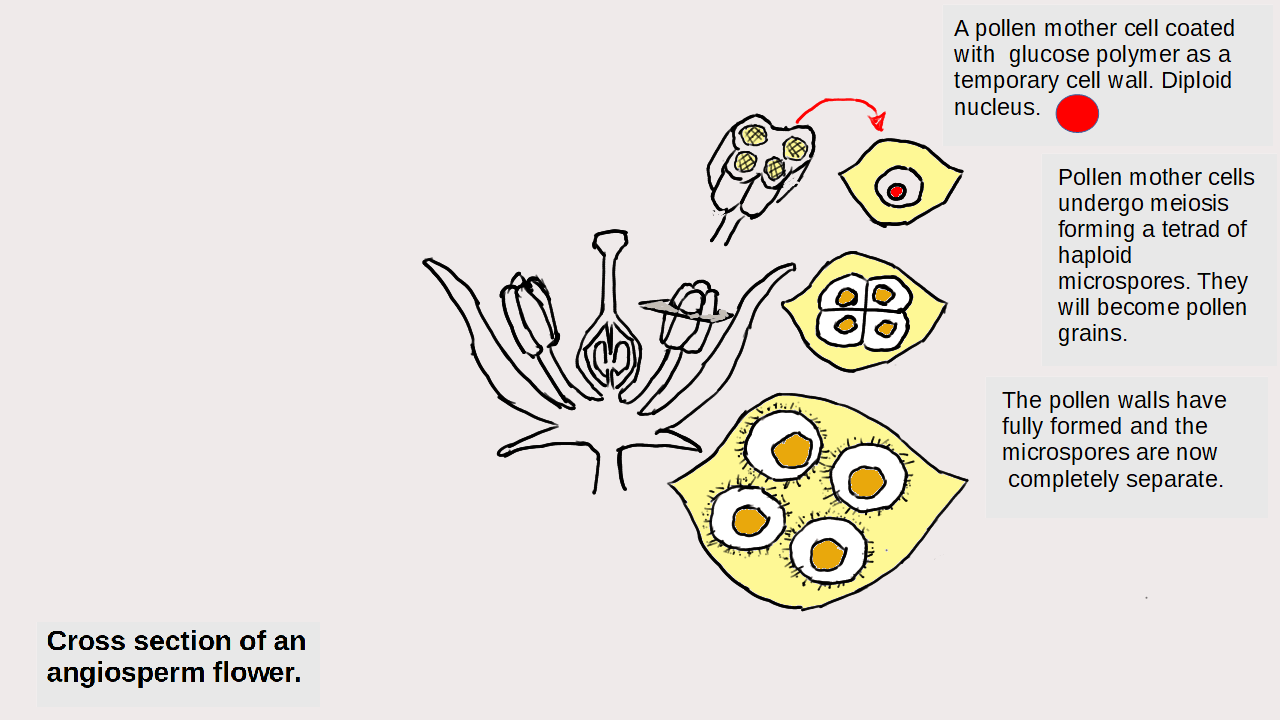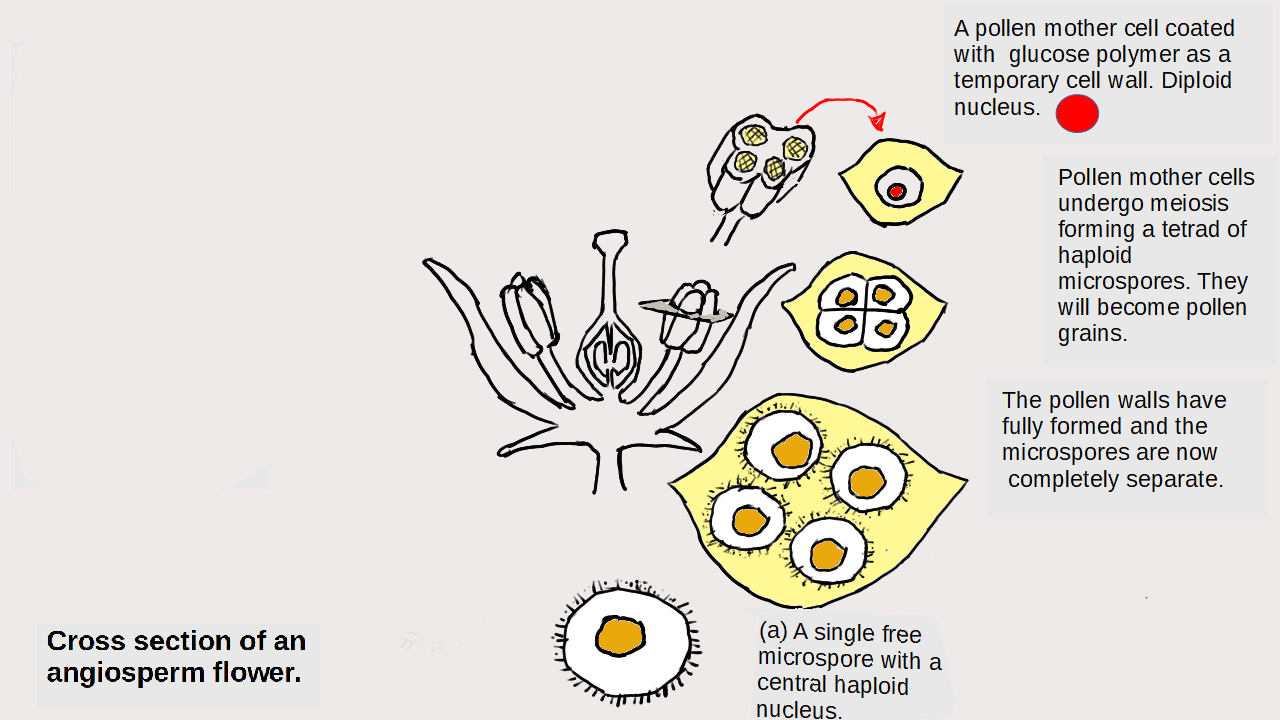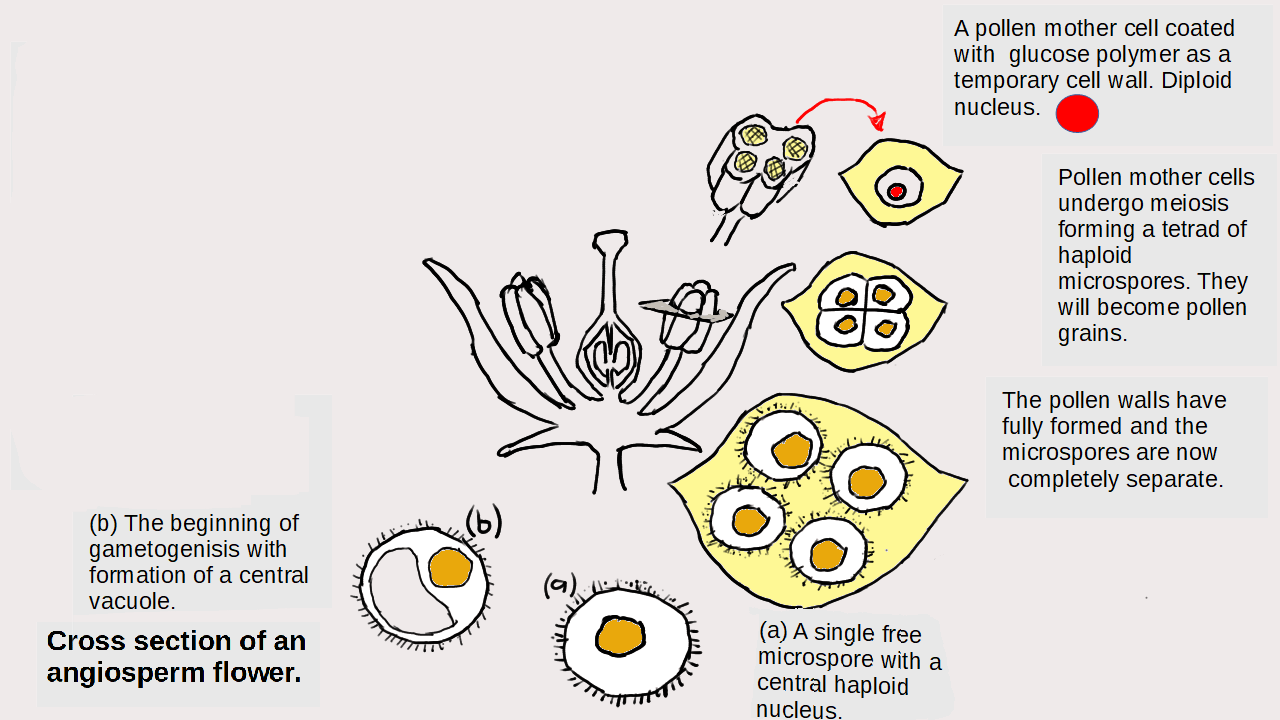
Particles and Thermochemistry level 3
Demonstrate understanding of thermochemical principles and the properties of particles and substances
Lewis structures also known as Lewis diagrams are diagrams that show the bonding between atoms of a molecule and lone pairs or non bonding electrons. Using these structures it is possible to predict the shapes of covalent molecules.
Working out Lewis structures involves the use of an arcane set of rules the only justification for which is that they appear in chemistry text books and examinations.
There is an app for this. Learn how to construct the diagrams but use the app to check answers. It works by plugging the formula. You don”t need to use subscripts.
For example
methane CH4
sulphuric acid H2SO4
You can also type in the name methane.