Acids and bases AS90944 year 11 revision.
Homework
End of term exams are looming. We revised the atomic structure aspects of this topic in class today and you may choose how you do your exam revision homework.
1) Print the practise test paper out at home and answer the questions on the printout
2) Download the practise paper onto your computer and answer the questions separately on your own paper.
3) Flick through the practise paper questions on the blog and answer them on your own paper.
DO QUESTION 1 TONIGHT
Practise paper
A look at the periodic table
The Periodic Table of all the elements in the universe left after recycling through supernova on Prezi
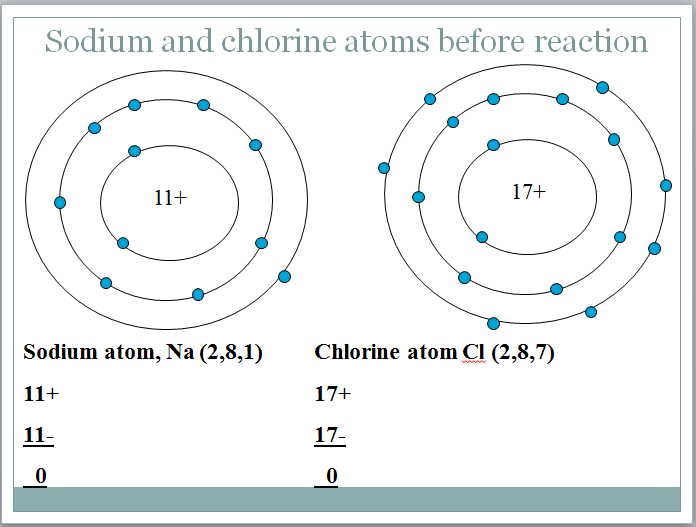
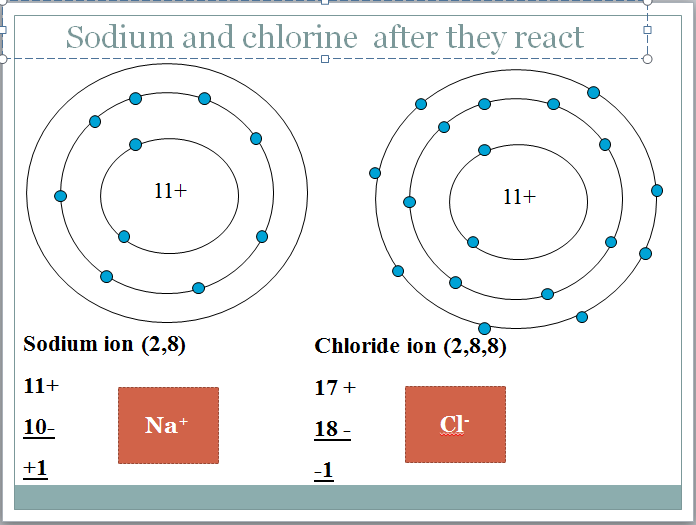
The reaction between sodium and chlorine to produce sodium ions and chloride ions. Atoms gain lose or share electrons to end up with a full outer shell. Compounds containing ions are called ionic compounds.