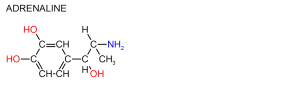Organic Chemistry Tutorial: Structural geometrical and optical isomerism
Many Biological molecules are optically active. Optically active organic molecules rotate plane polarised light. To be optically active they must have at least one asymetric carbon in their structure. Most optically active biological molecules rotate light to the left. Optical isomers are called enantiomers and they are non superimposable mirror images of each other. Enantiomers are identical, the only difference between them being the direction in which they rotate light. Special proceedures are used to separate them.
In nature only one enantiomer is biological active.