Predicting whether a precipitate will form.
Homework.
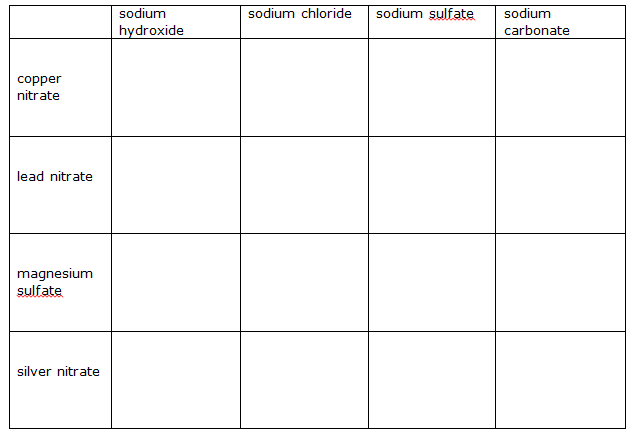
Head up homework with the date. Copy the table below into your book. Imagine mixing a solution from the left hand column with a solution from the top row. If a precipitate forms when the solutions are mixed write the name of the precipitate on the grid. If no precipitate results write “no precipitate” on the grid.
Use the solubility rules from your book or from the previous post.
The first example is done for you:
mixing copper nitrate and sodium hydroxide solutions.
step 1 write the word equation and switch the names around
copper nitrate + sodium hydroxide ——-> copper hydroxide + sodium nitrate.
Step 2 are any of the products insoluble
All hydroxides apart from sodium and potassium are water insoluble. Copper hydroxide is insoluble and a precipitate is formed.
write on the grid in the first space copper hydroxide precipitates.