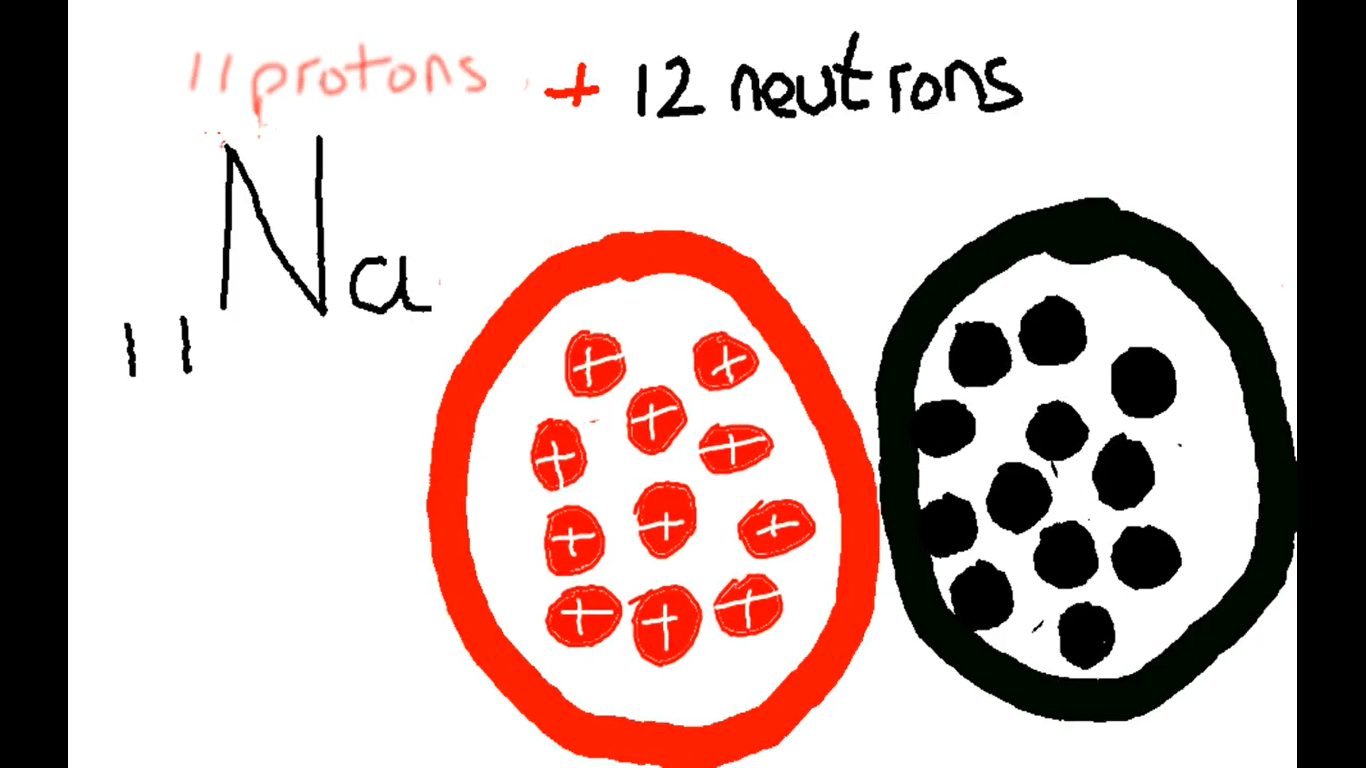
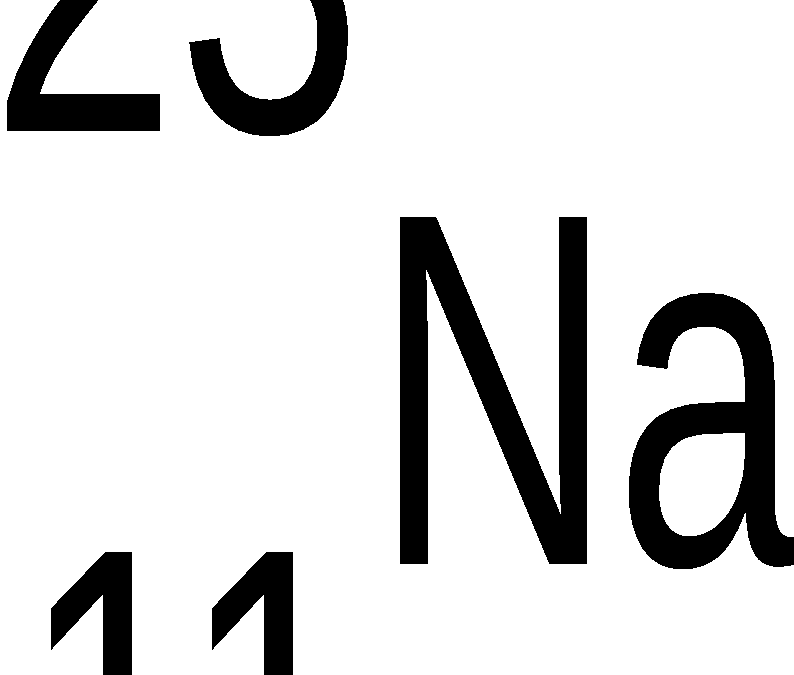Solids liquids and gasses, a story in three parts.
Part 1- tiny particles.
Food colouring spreads out when it is dropped in water.
Rotorua can smell a bit when gasses seep into the air from deep underground.
You know very quickly when someone has used Lynx in the classroom.
How can matter spread out so easily. It must be made of lots of tiny bits that can move about.
The first part of the theory is that all matter, everything that takes up space, consists of very tiny particles.

Solids liquids and gasses, the three main types of matter.
The concrete around the pool is definitely a solid.
The water in the pool is a liquid. The pool is dirty and there are also bits of solid present.
…….. but where is the gas in the picture?
Solids like concrete have a fixed shape.
Liquids can be poured and take the shape of their container. They can’t be compressed.
Gasses expand to fill up the space that contains them. They can also be compressed into a smaller space like air pumped into a football.

Part 2 – spacing and movement.
According to our theory everything consists of small particles.
How close to each other do particles approach?
How fast are they moving and do they collide?
How does our theory explain the behaviour of solids liquids and gasses?
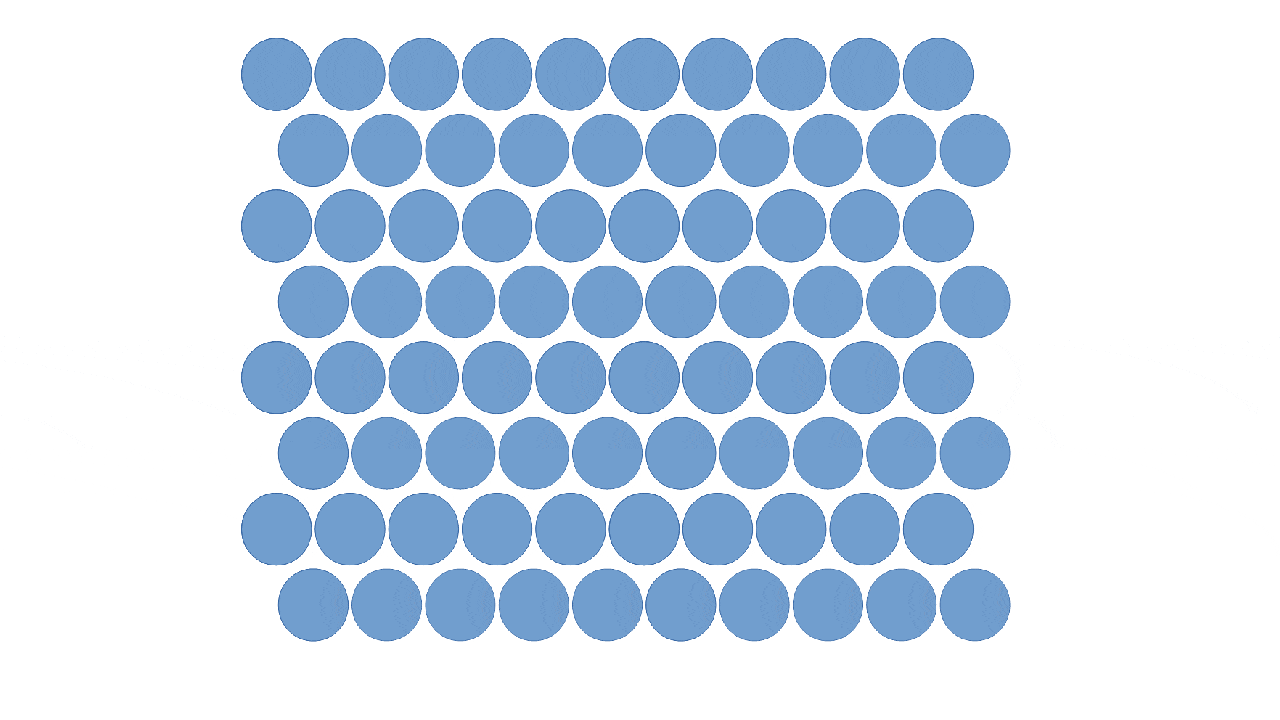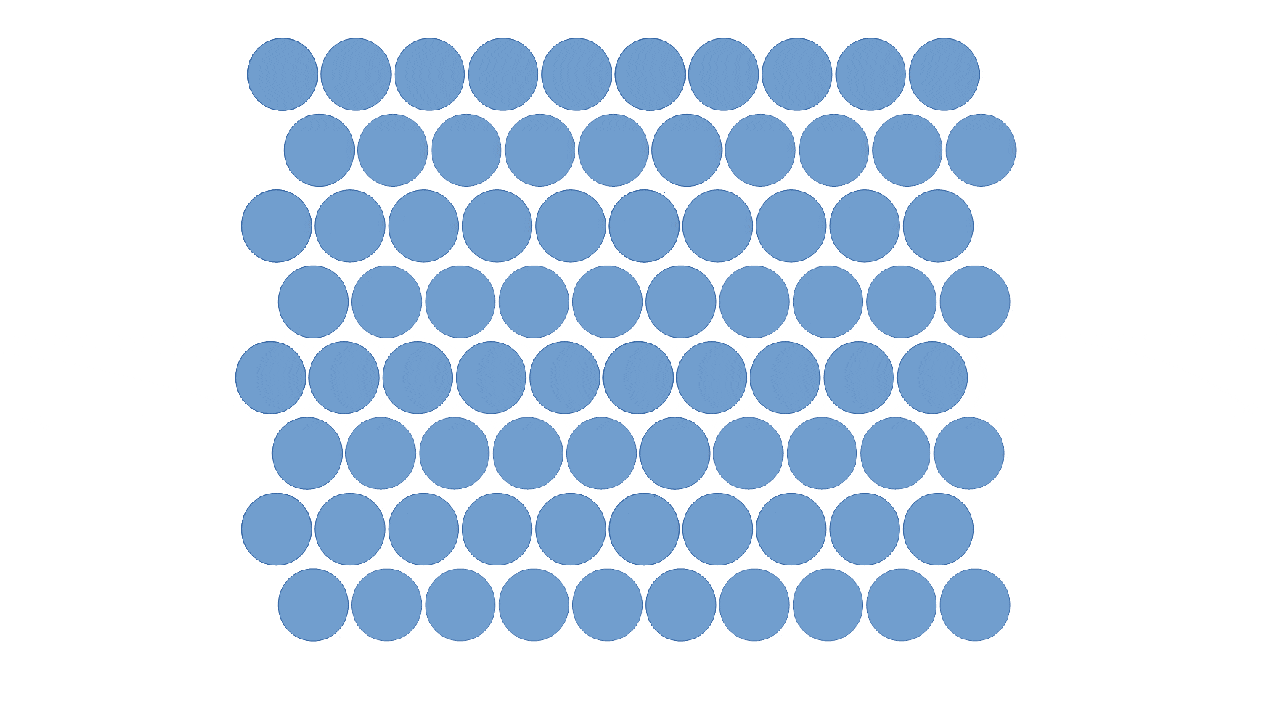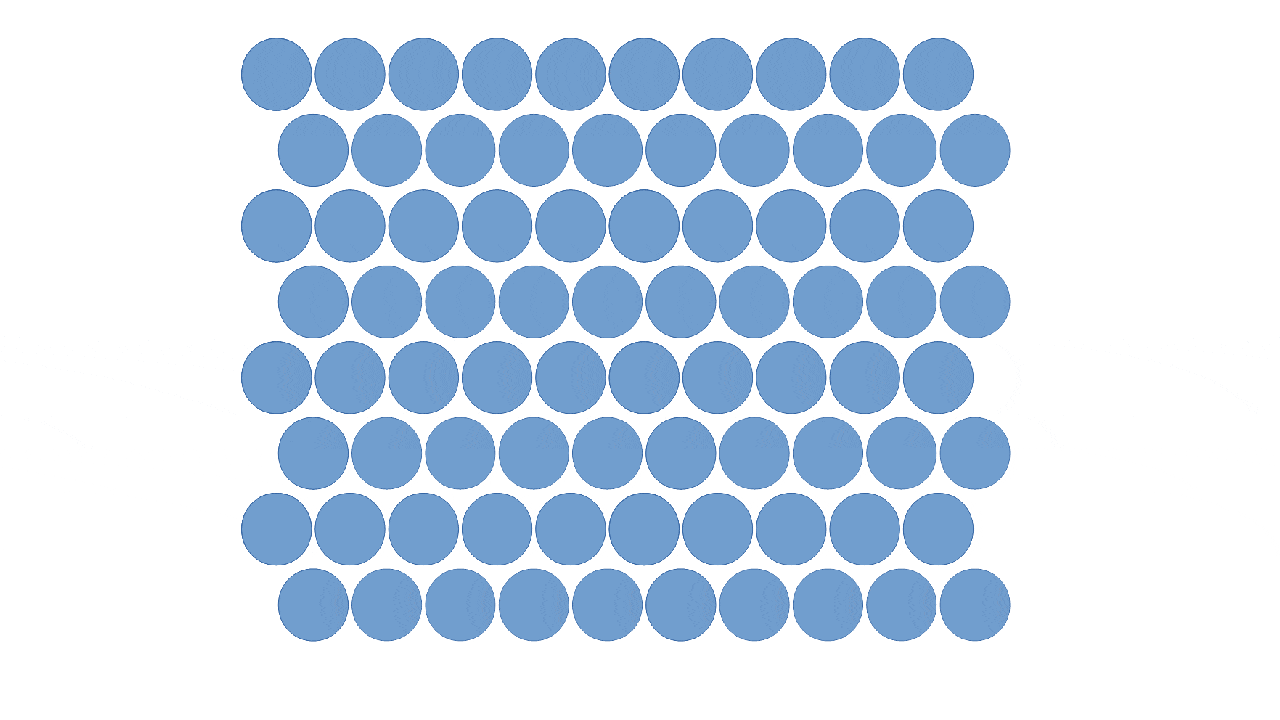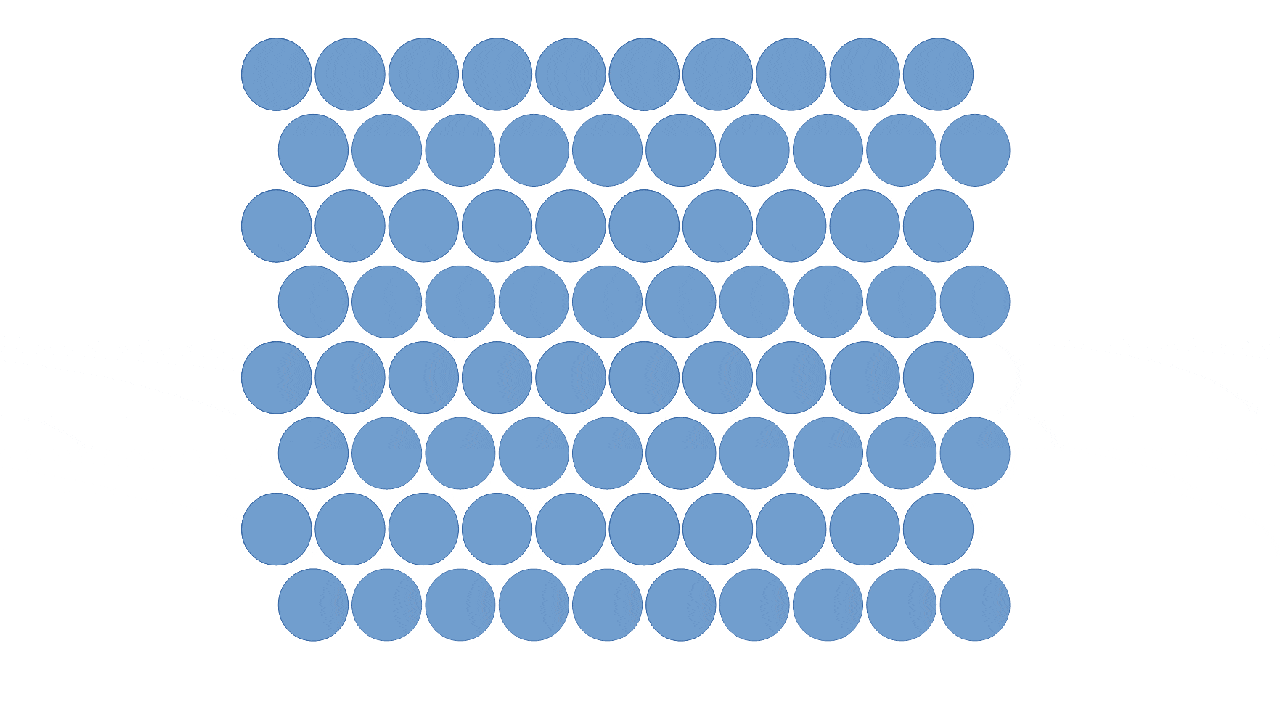
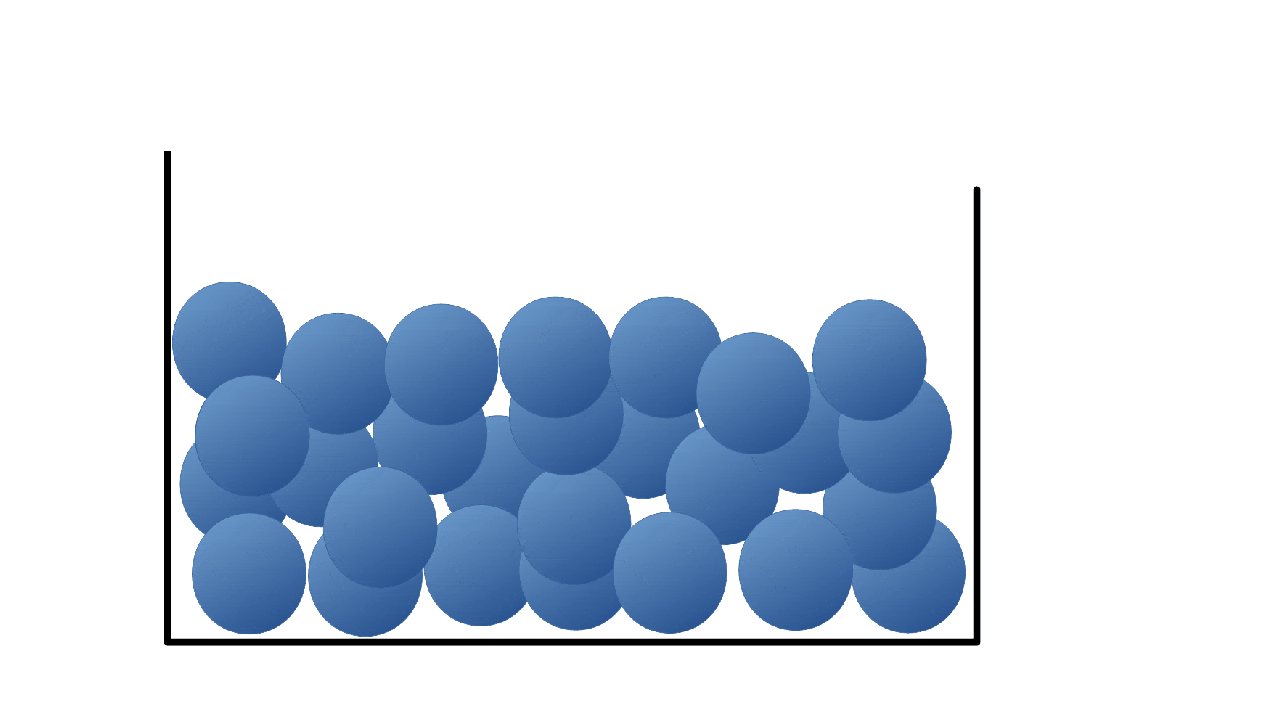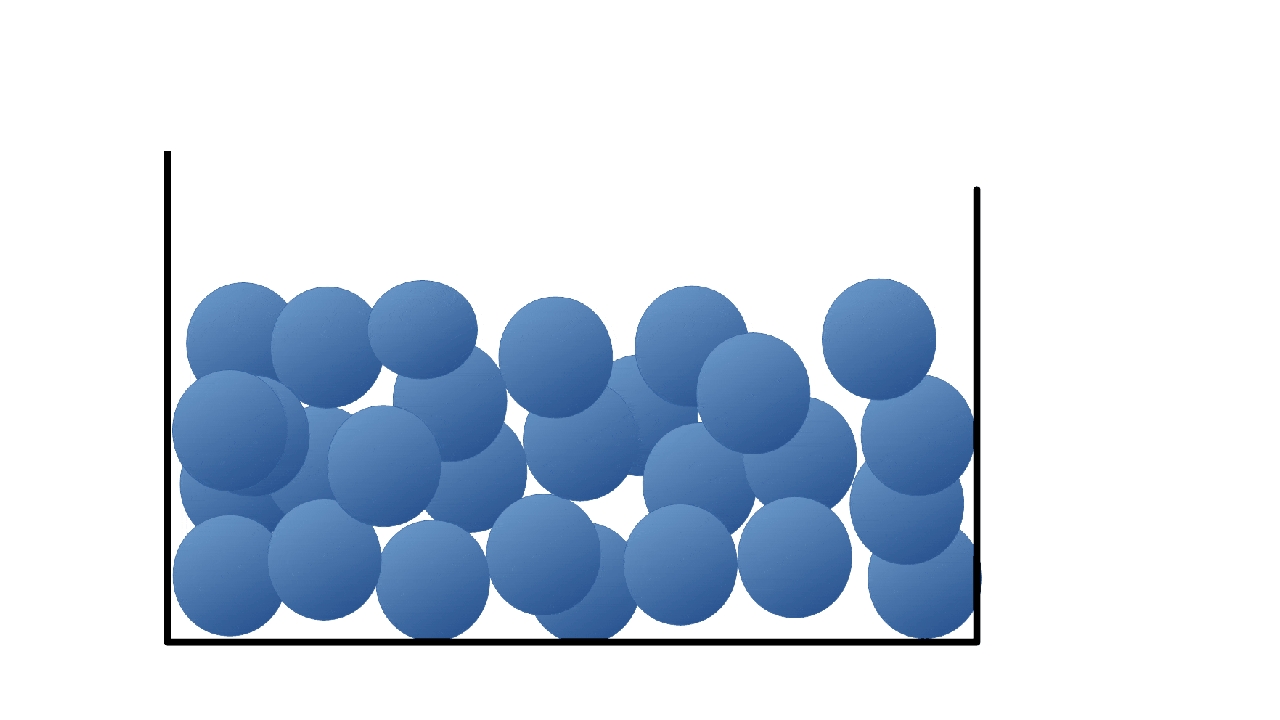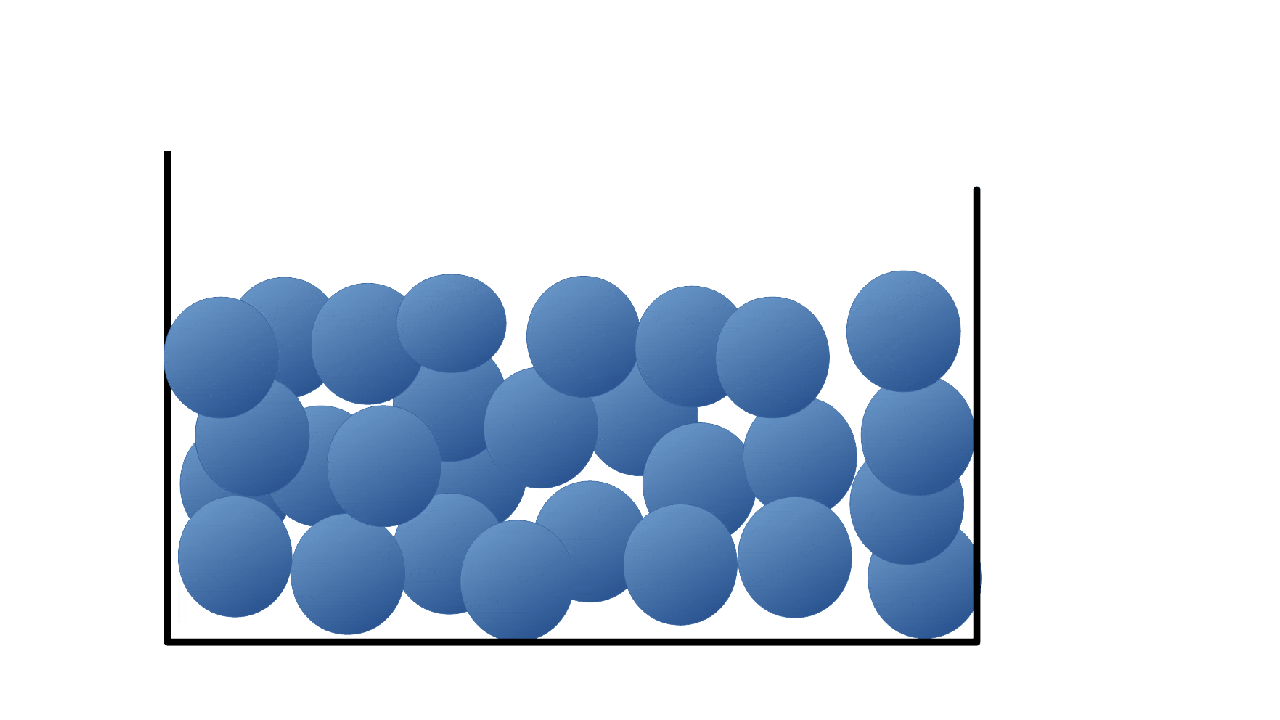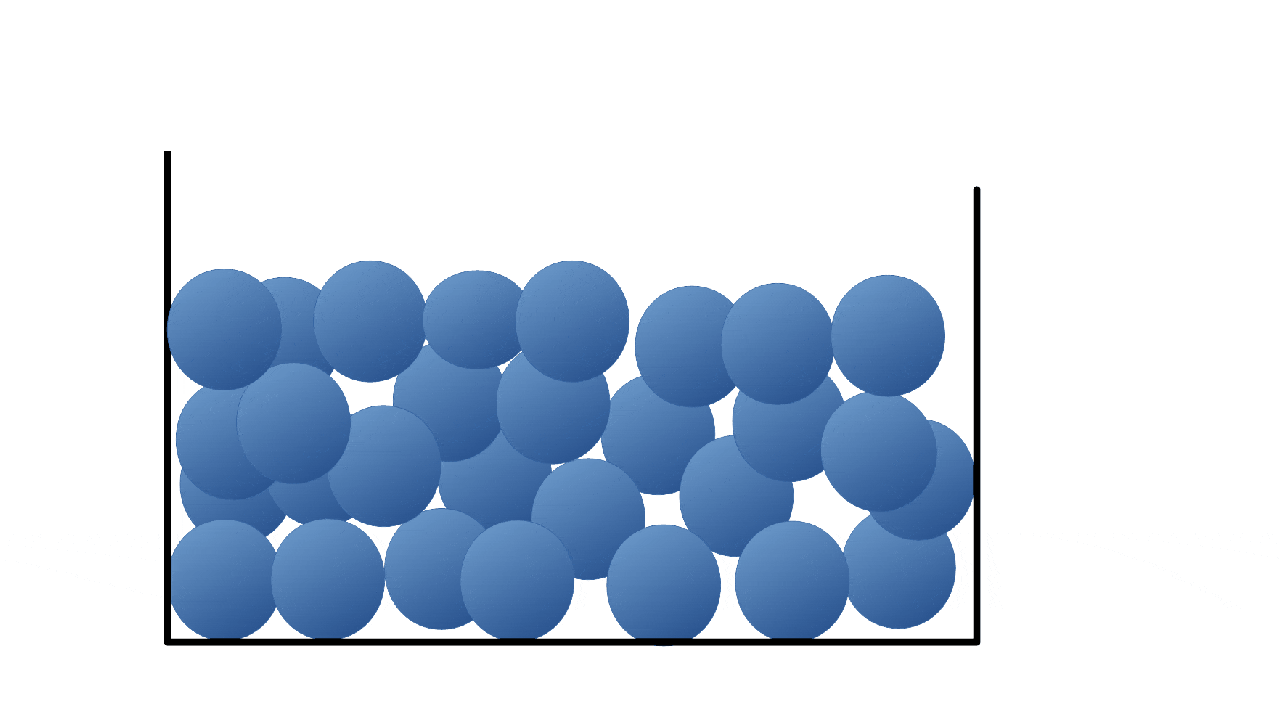
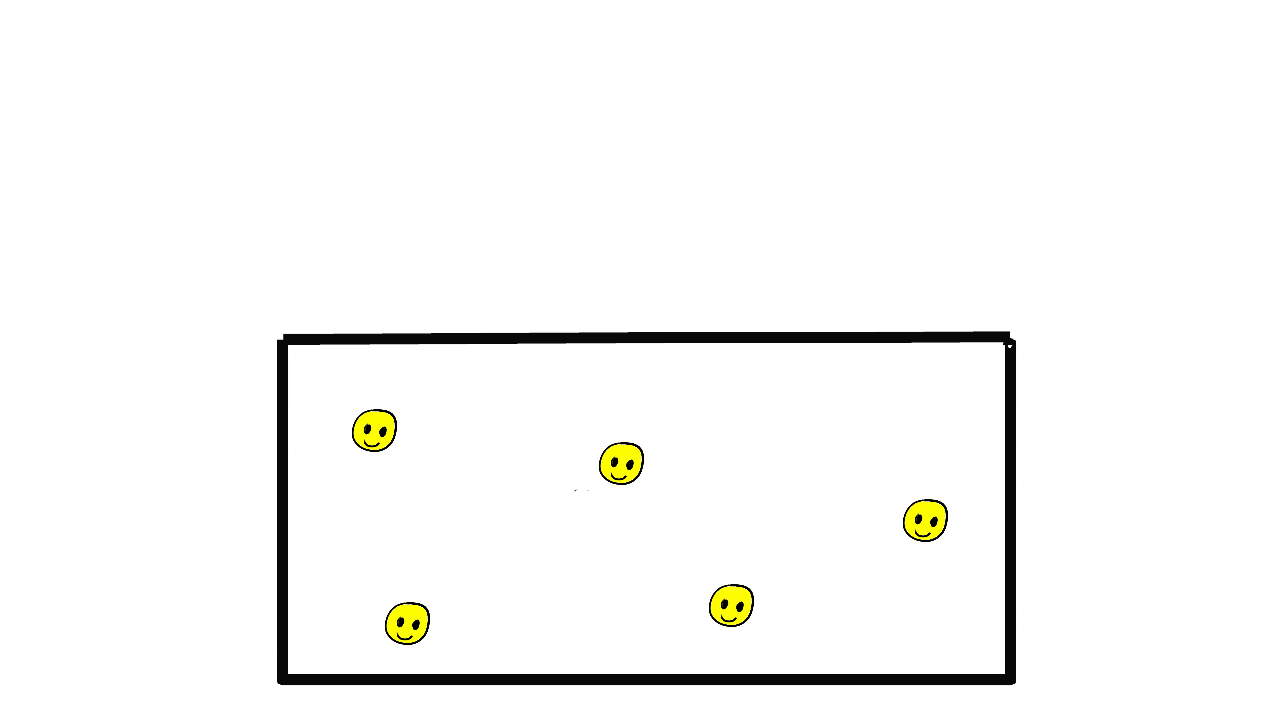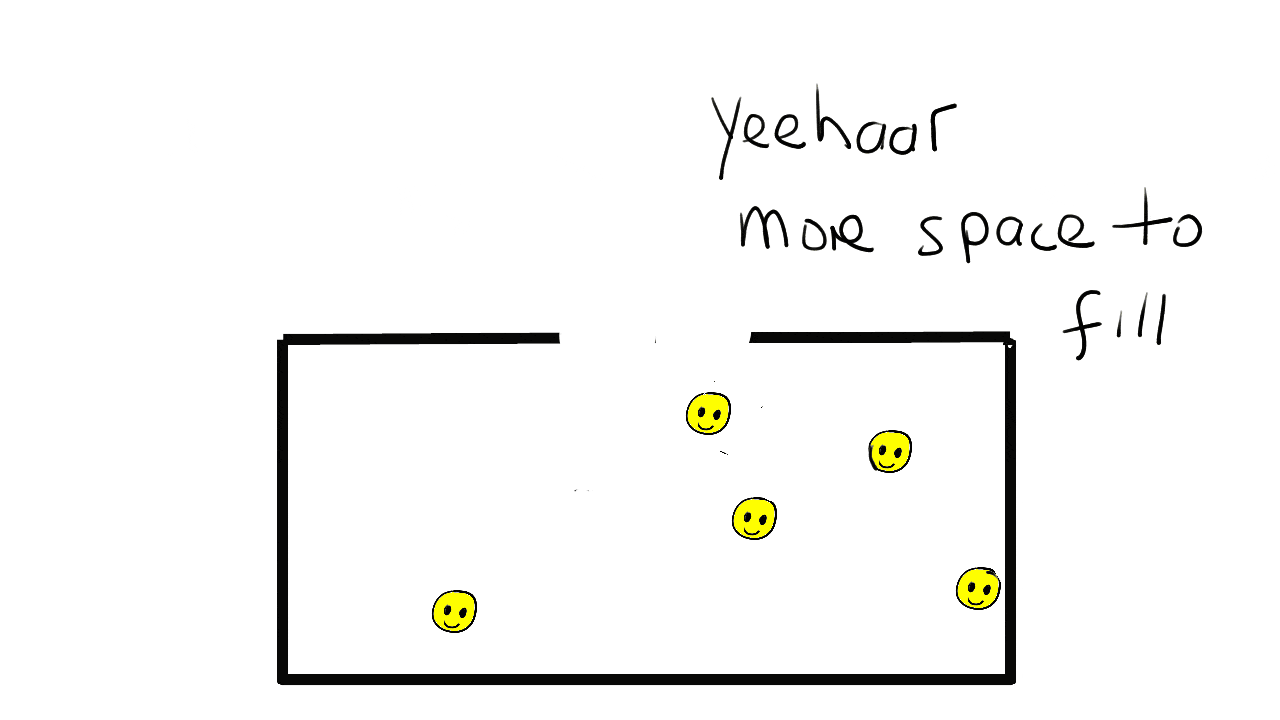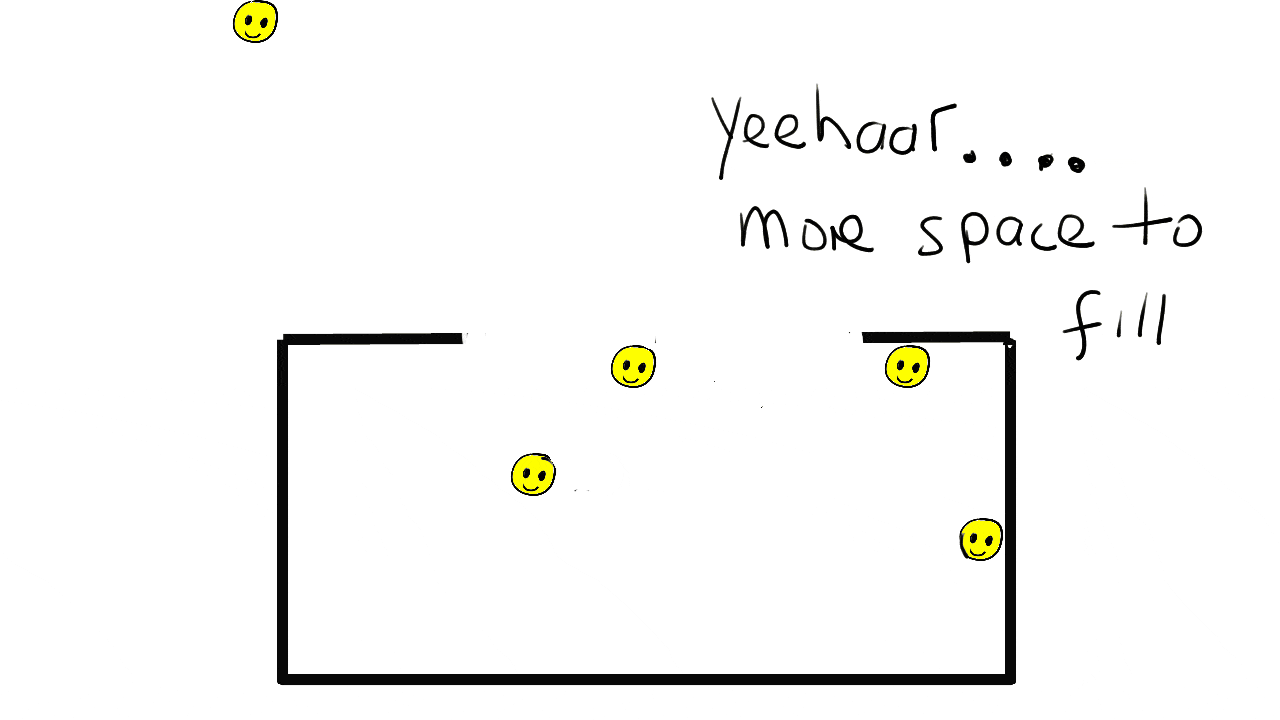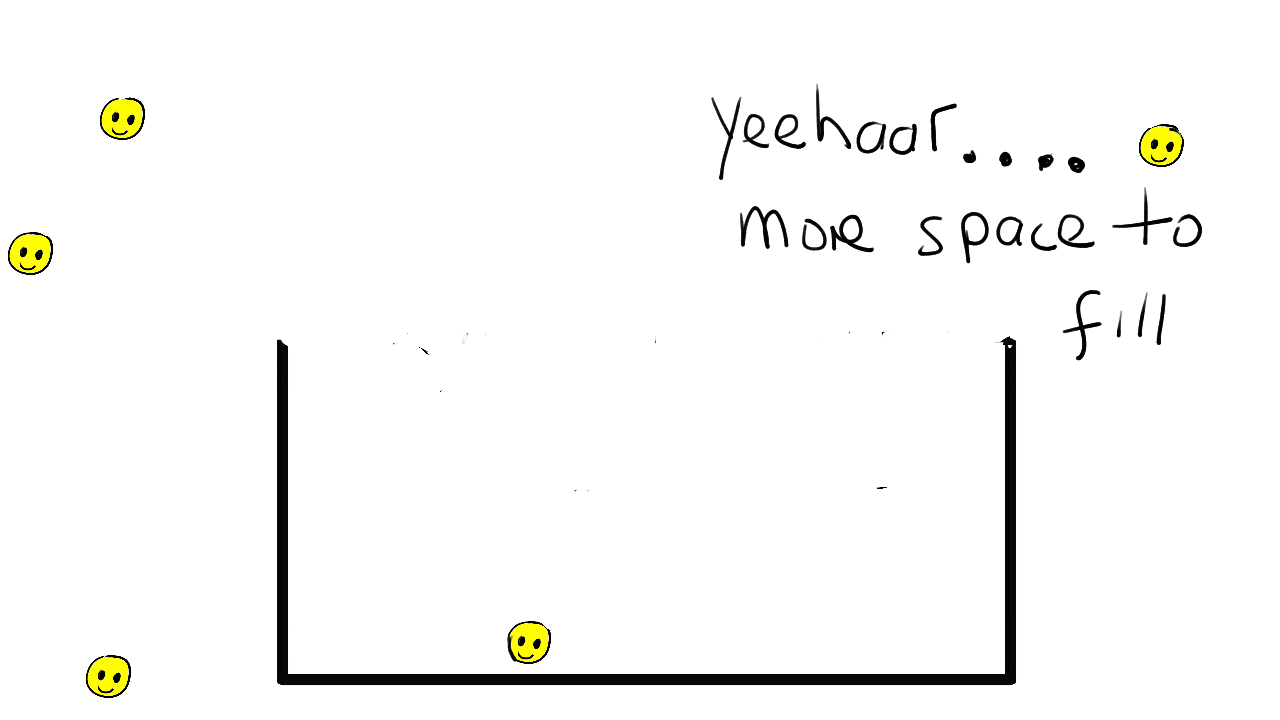
Solids
Particles are close together in fixed positions.
Particles can move slightly by vibrating.
Liquids
Particles are close together but they are free to move around each other and change their position.
Gasses
The particles are much more widely spaced in a gas. They can move around freely in the container bouncing of walls and each other.
Solids liquids and gasses.
When you heat a solid to a high enough temperature it melts and becomes liquid.
When you heat a liquid to a high enough temperature it boils and becomes a gas.

Part 3 – solids can become liquids and liquids can become gasses or the other way around.
The third part of the theory. When a substance is heated the particles pick up extra energy. The particles vibrate or move faster.
- As the particles in the solid vibrate faster they eventually start to break away from each other and move freely.The solid becomes liquid.
- As the particles in the liquid warm up they move past each with increasing speed. Eventually at the boiling point they have enough heat energy and are moving fast enough to break free completely and become a gas.
- Melting, when a solid becomes liquid on heating.
- Freezing, when a liquid becomes solid on cooling.
- Boiling, when a liquid becomes a gas at its boiling point.
- Condensing, when a gas becomes a liquid