NCEA Level 2 Chemistry: rates of reaction, strong and weak acids
Rate of reaction Research project
For a detailed look at how this practical is carried out visit this link in the blog
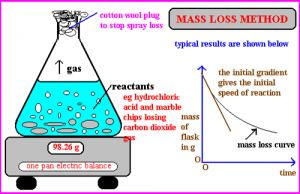
mass loss
When a gas is given off during a chemical reaction the rate can be measured by weight loss on a pan balance.
Decide how you will compare the rates of reaction between 2.0 and 1.0 mole per litre hydrochloric acid with marble chips. Compare rates of reaction between 2.0 moles per litre hydrochloric and ethanoic acids with marble chips.
Gather data (use 50.0 ml samples of the acids for your investigations) and plot your results graphically.
A follow up investigation might look at the pH of sequentially diluted solutions of ethanoic and hydrochloric acids (2.0, 1.0, 0.1, 0.01, 0.001 moles per litre)
Research project write-up.
Do a brief experimental method and graph you results using a spreadsheet
Present your results on word document or powerpoint. Discuss your findings in terms of :
-
species in solution
-
observed rates of reaction
-
total mass of carbon dioxide released (starting with the same amount of acid)
-
the strengths of the acids and equilibrium concentrations
-
the pH of both acids at different concentrations
As a follow up to your research track the pH changes that occur during these reactions and discuss.
